The actual matrix for geochemical processes in sediments is water, which is ubiquitous in sedimentary basins where hydrocarbons form and accumulate. Water occurs as a free aqueous solution in rock pores and fractures, as a surface-coating water film around mineral grains (so-called irreducible water), as part of multi-component gases, or bound to/into hydrated minerals. As such, the aqueous microsphere in the subsurface is the reactor in which a seemingly limitless number of geochemical processes may take place, either microbially-mediated or abiotically, at all temperatures. Geochemical processes involved in hydrocarbon systems are predominantly hydrogeochemical reactions because water is either a reactant or a reaction product in most of them. This includes biogenic methane generation during early diagenesis; oil formation and its degradation; porosity creation in carbonate reservoir rocks by corrosive fluids; thermochemical sulphate reduction; and scale formation resulting from seawater injection. Moreover, aqueous solutions in sediments are the matrix for diffusive and/or advective mass transport.
Appropriate computerised calculations (numerical simulations of reactive mass transport) are needed to make quantitative statements about the ‘where, when, and how much’ of water-rock-gas interactions and their effects on reservoir properties. However, traditional dogma about (hydro)geochemical processes in sedimentary basins still dominates; the focus is on isolated, selected reactions, such as abiotic sulphate reduction at high temperatures. Less attention is paid to the significance of water-rock-gas interactions as a complex web and of their coupling with mass transport processes, ranging from the micro to the macro scale in time and space. It is therefore mandatory to describe and to quantify the interacting hydrogeochemical reactions and the concurrent in-reservoir mass transport processes in four dimensions.
Basic Component in Hydrocarbon Systems
In hydrocarbon-bearing or other sedimentary systems rich in organic carbon, the driving force of complex organic-inorganic interactions is the conversion of thermodynamically labile organic compounds into reactive inorganic components (e.g., CO2, H+). Such interactions are widely known; they occur from shallow to great depth, in siliciclastics or in carbonate rocks, in a variety of sedimentary basin settings and also in subduction zones – and crustal Martian rocks. In petroleum systems water is an essential and basic reactive component in process chains during the generation of hydrocarbons, and its presence controls migration patterns. Additionally, it is a matrix for complex, interrelated hydrogeochemical reactions not just during geological alteration, but also in oil production.
However, such processes are dependent on a number of other controls. Temperature and pressure are exemplary basic physical parameters. The composition of the mineral phase assemblage, the type of water (e.g., the content of total dissolved solids, pH, EH) and of co-existing gases (e.g., CO2 partial pressure), and the type and the amount of soluble hydrocarbons, are all further important controls.
Several by-products formed during these interrelated processes are of great importance for reservoir studies. Changes in the mineral matrix may affect reservoir properties of oil and gas fields due to dissolution of unstable minerals directly coupled with precipitation of new and stable minerals, or to swelling of expandable clay minerals. This is often linked to lower API grades of oil, and also to changing gas:oil ratio and gas composition. Numerical simulations enable us to quantitatively describe the results of such reactive mass transport processes; for example to predict a potential hydrogen sulphide (H2S) and carbon dioxide (CO2) risk ahead of drilling. There are additional topics which relate to such conceptual approaches, such as seawater and CO2 injection for EOR or sequestration.
Organic-Inorganic Interactions: From Shallow to Deep
During sedimentation, degradable compounds of the sedimentary organic matter are converted to water-soluble reaction products like methane, carbon dioxide, molecular hydrogen and acetic acid (plus further low-molecular carboxylic acids). At low-temperature conditions in most marine sedimentary systems, early biogenic methane may be dissolved in the pore water until saturation; subsequently, a free multi-component, methane-bearing gas forms which, at suitable temperature/depth conditions, may be converted into solid methane hydrate. At greater depth, with a temperature of 40°C, a second peak of biogenic methane generation occurs. Again, numerical simulations enable us to quantitatively describe the results of such reactive mass transport processes evolving in dynamic systems like growing sediment columns.
Such basic processes are driven by the thermodynamically labile character of organic compounds and their understanding may be used to also understand processes at greater depth. In general, due to thermodynamic constraints, all organic-inorganic interactions, and thereby induced water-rock-gas interactions, are more or less similar from shallow to great depths. Water acts as the solvent for organic and inorganic reactants, and, notably, it may also be produced or consumed by such interactions. The chemical composition of aqueous solutions and the composition of coexisting multi-component gases and mineral assemblages change as inevitable consequences of chemical thermodynamics: the involved inorganic reactions tend to reach stable, thermodynamically-defined equilibrium conditions. In contrast, the degradation processes of organic matter proceed as irreversible, kinetically controlled reactions. The reactive inorganic products of such organic matter degradation (e.g., CO2, H+) drive new inorganic balances.
At greater depths, sedimentary organic matter can be converted to kerogen and release crude oil and natural gas. On both the micro and macro scale there are abundant interfaces in oil and gas reservoirs at greater depth where organic-inorganic interactions may lead to CO2 and H2S formation. These are the oil-water transition zone (OWTZ) or the reservoir-seal contact; if anhydrite forms the seal, reactive interfaces may occur inside anhydrite-bearing carbonate reservoirs.
How to Predict CO2?
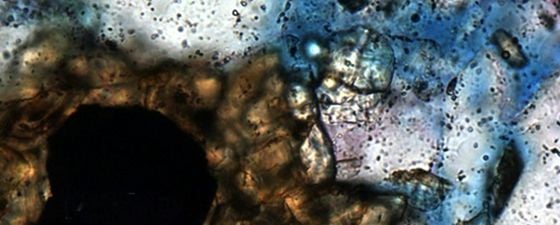
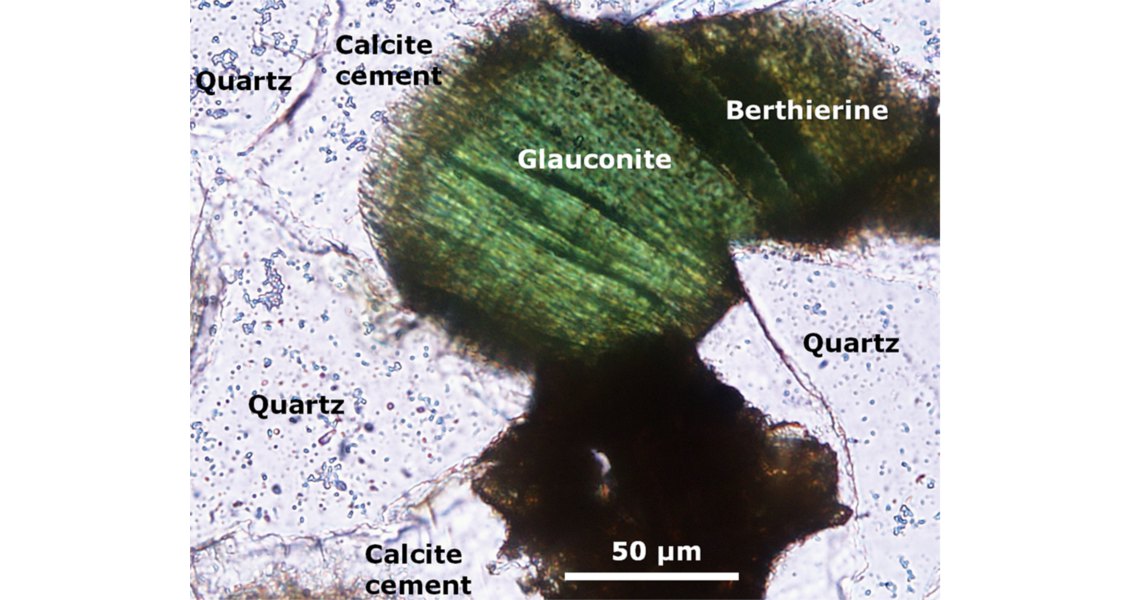
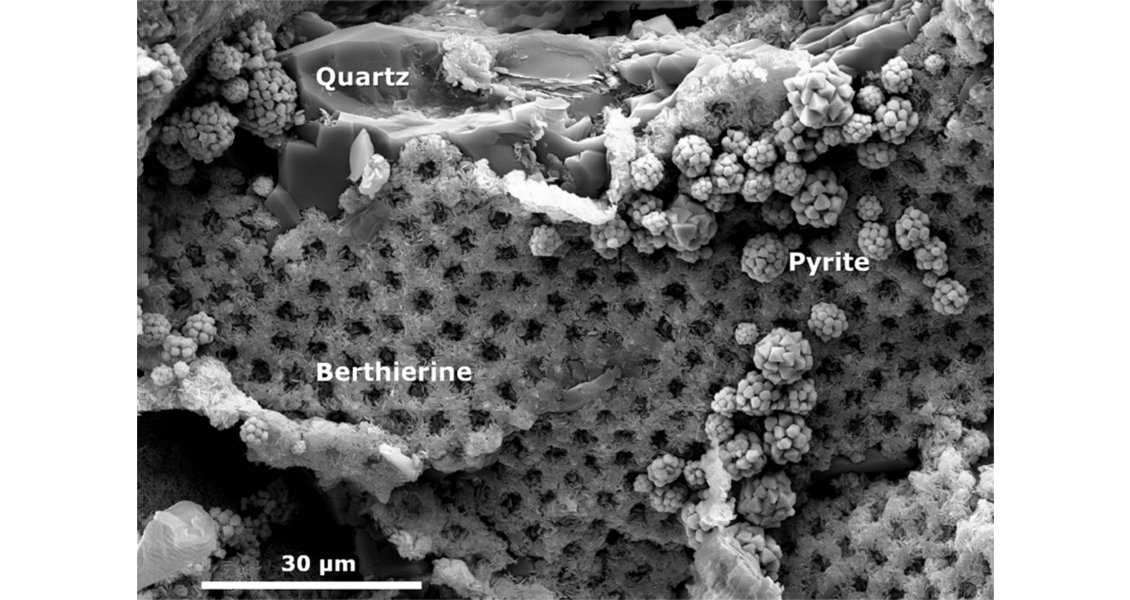
The OWTZ is the main interface where oil degradation and coupled organic-inorganic interactions take place. However, water-wet, oil-stained reservoir intervals also represent microspheres for such reactions, together with intervals where irreducible water mainly occurs around mineral grains. As a result, CO2 as a main degradation product (besides methane) is being released into the formation water and can change the hydrogeochemical conditions. Such changes are strongly related to the rock matrix, and sensitive minerals like feldspar, carbonate or glauconite control the fate and behaviour of CO2 by, for example, determining whether dissolved CO2 may be trapped as carbonate minerals or not. Glauconite may dissolve as result of oil degradation, and minerals like berthierine can form.
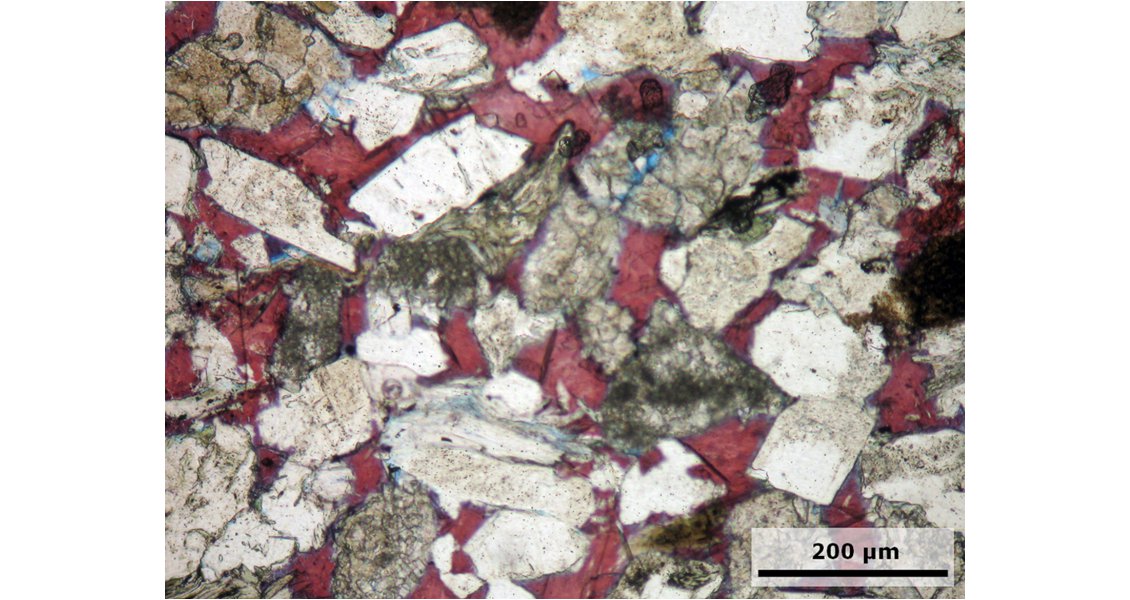
In addition, the feldspar composition and content in a reservoir rock mineral assemblage determine further reactions related to CO2, including whether newly formed kaolinite decreases porosity and permeability. Under conditions of intense oil degradation, anorthite and albite are the least stable feldspars; their dissolution may lead to the formation of calcite or other carbonates, and additional sodium-bearing carbonates may form. However, if the CO2-buffering capacity of the reservoir rock matrix and reservoir fluid is exhausted, CO2 may occur as a gas and pollute the reservoir gas. To investigate the relation between the CO2 behaviour and different minerals, appropriate numerical simulation approaches have been successfully tested for North Sea oil fields.
H2S in Your Play?
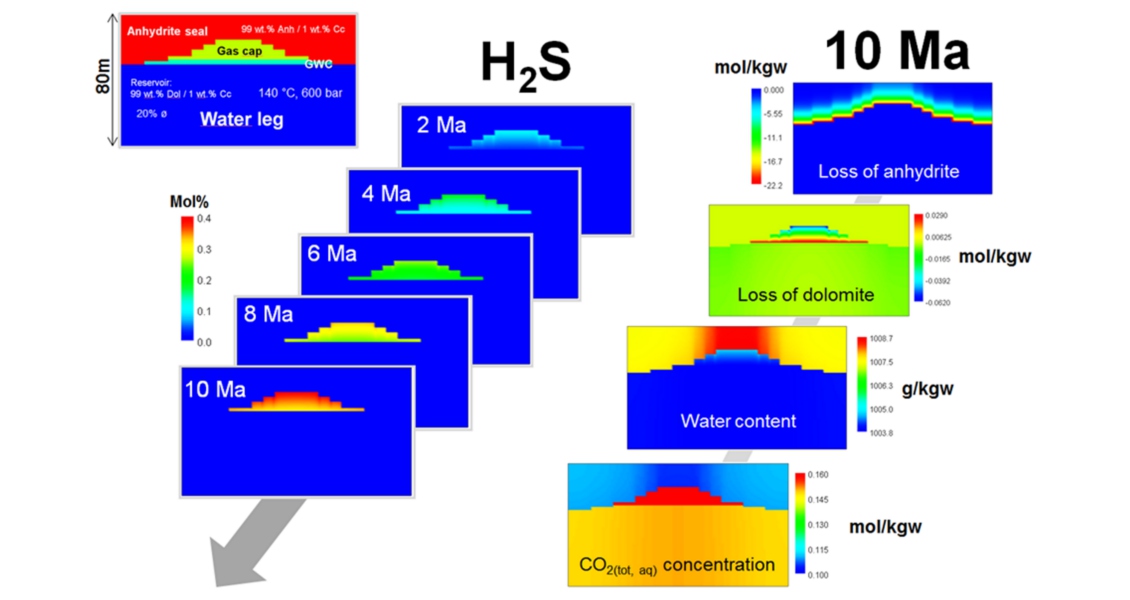
H2S is toxic, corrosive and lowers hydrocarbon value. For example, production from deep anhydrite-sealed carbonate reservoirs exposed to higher temperature and pressure conditions will face more aggressive corrosion environments, inevitably increasing risks and costs resulting from thermochemical sulphate reduction (TSR). But under which conditions does H2S actually form in a reservoir, if it is not sourced from deeper horizons such as deep magmatic rocks? And which are the basic considerations for numerical 3D simulations with temporal resolution? Again, an interface is the reactive site for a complex reaction web.
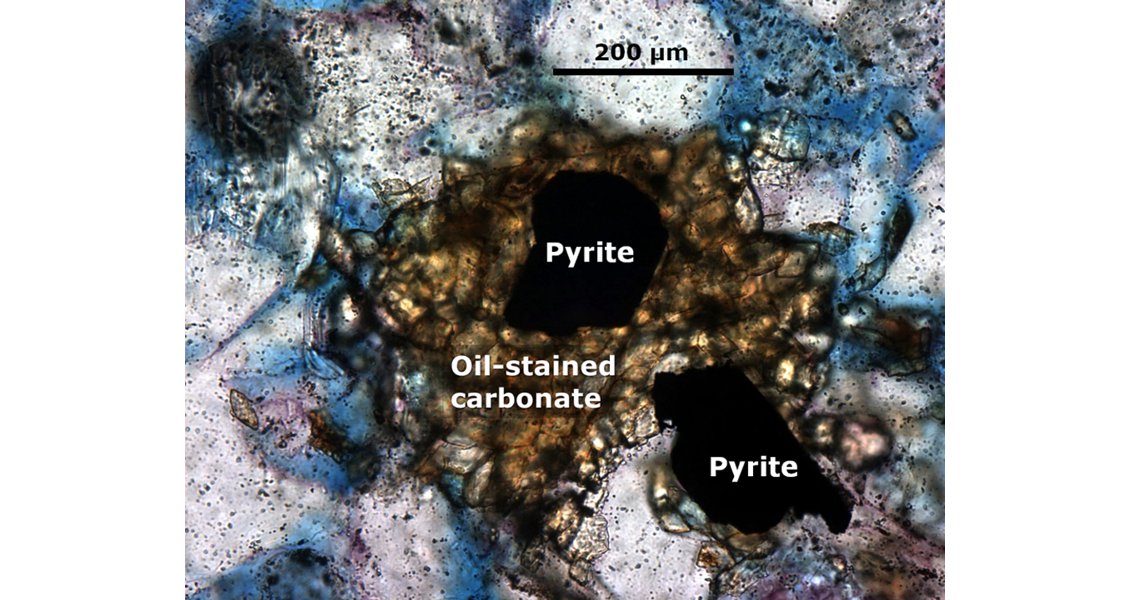
Besides the OWTZ, the reservoir/ (anhydrite) seal contact is an interface where aqueous components such as sulphate, methane and/or other short-chained hydrocarbons react in a complex and self-reinforcing reaction web. As only small amounts of water (e.g., irreducible water) are needed to dissolve anhydrite, hydrocarbons from the gas cap interact with dissolved, anhydrite-derived sulphate and trigger H2S formation. This process resembles, in principle, H2S formation during early diagenesis, but is an abiotic sulphate reduction at high temperatures (>100°C). However, this basic reaction is only a fragment of a broader reaction web, which also includes formation of elemental sulphur, pyrite, and calcite as mineralised by-products. Important, but often underestimated, is that one net product within the TSR reaction web – water, which is the actual matrix of all interrelated reactions building this integrated TSR web. Furthermore, mass transport of dissolved reactants in the reservoir plays a crucial role in TSR. A new approach to resolve H2S formation as one of the TSR products in time and space was recently presented by Fu and co-authors, which enables the prediction of this process in frontier regions.
Cutting-edge Concepts
Water is everywhere in the subsurface. Its hydrogeochemical composition stores information about the basin history and its manifold geochemical processes. Interdisciplinary concepts help to improve long-established methods and to analyse past processes, but also to forecast consequences, such as those induced by reservoir engineering. Analysing and retracing geological processes will help us to plan technical measures better, including storing CO2.
In short: by coupling petroleum geology with hydrogeochemistry we are able to enhance traditional methods with cutting-edge concepts.