It should be apparent from the title of this article that the author does not like the use of the word ‘photon’, which dates from 1926. In his view, there is no such thing as a photon. Only a comedy of errors and historical accidents led to its popularity among physicists and optical scientists … There are very good substitute words for ‘photon’, (e.g., ‘radiation’ or ‘light’).
– Willis E. Lamb Jr. (1913–2008), American Physicist and Winner of the Nobel Prize, in his article ‘Anti-Photon’ (1995)
Increased concentrations of greenhouse gases are warming Earth’s surface and troposphere. But more greenhouse gases seem to lower temperatures in the higher layers of the atmosphere: the stratosphere (above 15–20 km), the mesosphere (above 50 km), and the thermosphere (above 90 km). This cooling, often referred to as ‘stratospheric cooling’, is evident from measurements and considered to be one of the fingerprints of anthropogenic global warming. Here, we present observations. In a second part, we introduce the 1D window-grey radiation model of the atmosphere, which illustrates the physical essence of the mechanism by which a CO2 increase cools the stratosphere and mesosphere.
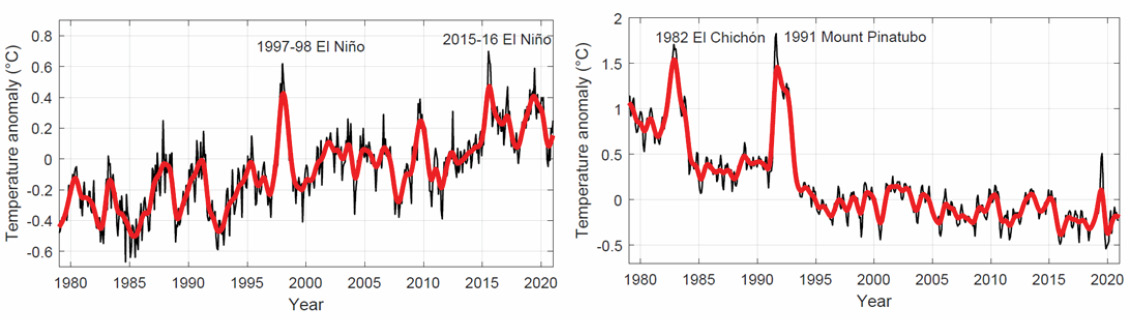
Figure 2 shows annual global temperature anomalies (with respect to 1981–2010) from NOAA polar orbiting satellites for lower troposphere (left) and stratosphere (right). The numbers, worked out from the satellite measurements by scientists at the University of Alabama in Huntsville (UAH), express the difference between the average temperature in a given month and the long-term average between 1981 and 2010 for that month. The differences are called anomalies and they indicate how temperature is changing over time. On Earth’s surface, where we live, and in the troposphere, global warming means long-term increases in temperatures. About 80% of the mass in our atmosphere resides in the troposphere, including most of the greenhouse gases. Since 1979, the lower troposphere has warmed by 0.14 degrees per decade (see Figure 3).
If you lived in the stratosphere, around 25 km above the Earth’s surface, you would have experienced cooling temperatures over the past few decades.
If you think this could compensate for global warming, you are wrong. The graphs in Figure 2 show that stratospheric cooling has not neutralised the warming trend of the troposphere. What is going on exactly?
The answer is twofold: the depletion of stratospheric ozone is probably the main driver of the cooling in the lower stratosphere. In the middle and upper stratosphere (and beyond), the CO2 increase is believed to be the most important reason for the temperature decrease.
Ozone Depletion
Stratospheric cooling over the past 50 years can be attributed partially to human emissions of ozone-depleting substances (ODS) like chlorofluorocarbons (CFCs). CFCs were developed in the 1920s–1930s for use as refrigerants, solvents and aerosol-spray propellants. In the late 1950s, they were emitted in substantial quantities but have since 1987 been banned. Consequently, most ODS concentrations peaked in the 1990s and have been declining since then.
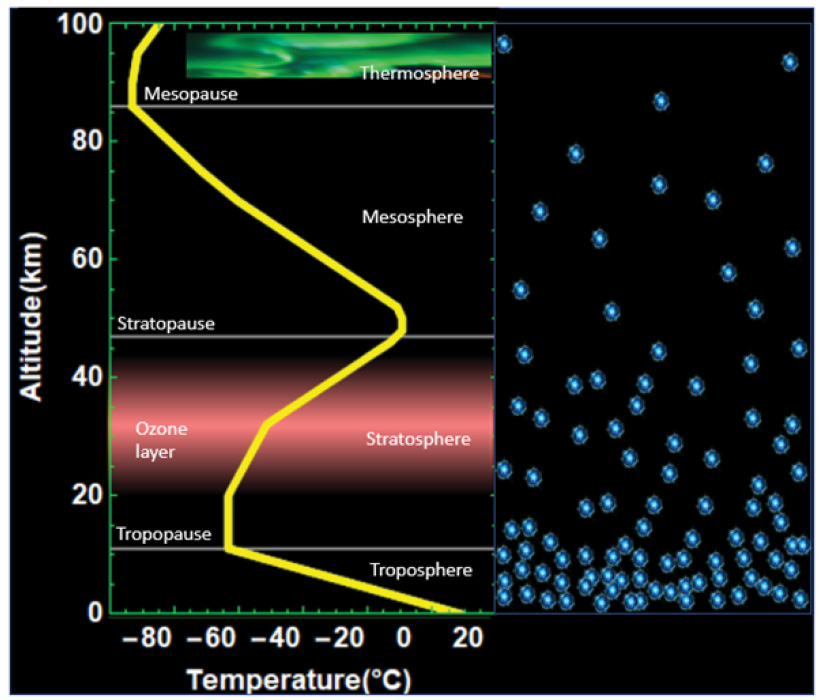
The ozone layer (Figure 4) resides in the stratosphere where it forms a protective blanket which shields life from solar ultraviolet radiation. Before ODS were regulated, the amount of ozone steadily decreased in the stratosphere. As the ozone decreased, the ability of the atmosphere to absorb solar radiation lessened. When less energy was absorbed, then the equilibrium stratospheric temperature lowered, resulting in a cooling of the stratosphere. Ozone also acts as a greenhouse gas in the lower stratosphere. Less ozone implies less absorption of infrared (IR) heat radiation and therefore less heat trapping.
Recent research demonstrates a stratospheric ozone recovery (see, e.g., Banerjee et al., 2020).
Increase in CO2
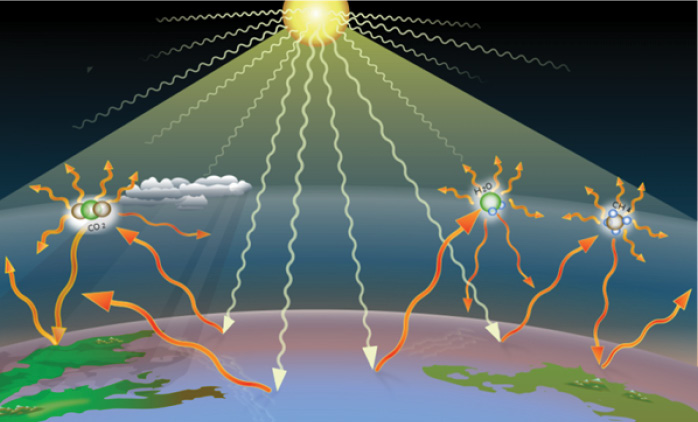
The second effect is more complex. To explain we need to go back to basics and look at previous learning in this series. We know that everything that has a temperature gives off electromagnetic radiation. Atmospheric radiation is the flow of electromagnetic energy between the Sun and the Earth’s surface (see Figure 5). It includes both shortwave solar radiation (sunlight) and terrestrial radiation. Terrestrial radiation, also called thermal infrared radiation (IR) or outgoing longwave radiation (OLR), is the electromagnetic radiation of wavelengths from 3–100 μm emitted from the Earth and its atmosphere. Generally, the atmosphere absorbs IR radiation due to the capacity of water vapour, carbon dioxide, and ozone to absorb IR energy. However, assuming no cloud cover, in the so-called infrared atmospheric window, from 8–13 μm, there is relatively little absorption of radiation by atmospheric gases.
The radiation processes in the atmosphere play a significant role in Earth’s energy budget, which describes the energy and radiation balance of the whole earth-atmosphere system. The downward radiation, due to the direct sunlight and the back radiation from the atmosphere, causes heating of the Earth’s surface. In this series, we have seen that the back radiation is the source of the atmospheric heating effect. The upward radiation ensures that the absorbed energy from the Sun and the terrestrial radiation can be rendered back to space. It ensures cooling and stabilises the Earth’s temperature.
CO2 molecules are very efficient at absorbing and re-emitting IR radiation. In the lower atmosphere, air molecules are tightly packed together. In ‘Part XI: How Earth’s IR Photons are Transferred in the Atmosphere in the Presence of CO2‘ (GEO ExPro Vol. 18, No. 1) we described the CO2 molecule’s absorption and emission process of photons (sorry, Lamb) in the troposphere. First, when photons having wavenumbers in the band around 667 cm-¹ (at a wavelength of 15 micrometres) are absorbed by CO2 molecules, only a very small percentage re-radiate radiation, in a random direction. The rest loses that energy to the surrounding bath of atmospheric molecules. In turn, the atmospheric molecules collide with CO2 molecules so that they get excited to a higher vibrational state. A very small percentage radiates new photons, again in a random direction, and the rest loses the energy by collision. The process repeats forth and back rapidly, and around 5–6% of the CO2 molecules present in the bath, radiate. This leads to the greenhouse effect, an overall heating of the troposphere.
In Part XI, and additionally, in ‘Part XIV: The Doom of a Photon on a Random Walk’ (GEO ExPro Vol. 19, No. 1) we calculated the mean free path, or the mean distance travelled by a photon before being absorbed. We found that a photon close to the centre of the absorption band, between 650–690 cm-¹ travels a distance of metres before being stopped. Concerning the photon’s travel upwards, the bottom of the troposphere might be seen as an impenetrable wall of CO2. However, even though CO2 has a strong photon-trapping band centred at around 667 cm-¹, the band has ‘wings’ that spread out 150–200 cm-¹ on either side. The probability of absorption, or the ability of a CO2 molecule to absorb a photon of a particular wavenumber, is represented by the CO2 absorption cross-section shown in Part XI. Photons having wavenumbers at the wings of the band are less likely to be absorbed and have mean free paths of hundreds of metres.
Further, in Part XIV we learnt that the absorption length is inversely proportional to the density of CO2 molecules at the location. Rising levels of CO2 add more CO2 molecules to participate in the process. The absorption at the centre of the band is already so strong that this band plays a small role in causing additional warming. However, more photons now are absorbed at wavenumbers in the wings of the band. More photons are re-radiated, causing heating of Earth’s surface and troposphere.
In the middle and upper atmosphere, air density and therefore the number of CO2 molecules is much less (Figure 4). Also here, around 5–6% of the CO2 molecules radiate in the bath of molecules. Due to the much lower number of CO2 molecules, the mean free path is significantly larger, and photons can travel a much longer way before being blocked.
The mean free path of a photon depends on the wavenumber (or frequency) of the photon and the CO2 concentration at the location of the photon. In Part XIV the definition of the mean free path led us to introduce the ‘number of layers’ of the exponential atmosphere that an IR photon has to traverse from Earth’s surface to outer space. The photons with wavenumbers in the centre of the absorption band are ‘seeing’ thousands of layers while photons with wavenumbers at the wings of the band are meeting only a few layers. In each ‘layer’ photons are absorbed and re-emitted in the way that we have described above. The more layers that are present, the less chance the photon has to escape to space.
In response to an increased CO2 level the mean free path of IR radiation is reduced, or equivalently, the atmosphere (in particular, the troposphere) is divided into more layers, each becoming thinner.
What is the Effect of CO2 Increase on the Stratosphere?
Since the number of layers has increased in the troposphere, and each layer absorbs and emits radiation, the upward radiation received from below must come from higher – and thus colder – levels of the troposphere (compared to the levels related to the pre-increase of CO2). While the CO2 molecules absorb radiation from the colder upper troposphere – and recalling that the CO2 gas emits according to the local temperature – the CO2 gas must emit radiation with the higher temperatures of the middle atmosphere.
Consequently, in the stratosphere and the levels above, the emission of energy (heat) becomes larger than the energy received from below by absorption. As a result, there is a net energy loss from the stratosphere and a resulting cooling. Therefore, increased CO2 concentrations impose an IR cooling tendency.
When the whole Earth system is in balance, Earth’s surface temperature has increased due to the increased CO2 concentration. When the surface and troposphere gradually warm, it results in an increased upward IR radiation at the tropopause which slightly warms the lower stratosphere. This warming however does not compensate for the initial cooling phase in the middle and upper atmosphere, so that the net effect of the CO2 increase is ‘stratospheric cooling’ when the planet is in overall equilibrium.
* Lasse Amundsen is a full-time employee of Equinor