Harnessing a natural process, mineralization of CO2 is the process by which primary and secondary minerals such as Mg, Fe, and Ca present in basalts interact with CO2-enriched fluids to convert it into solid carbonates.
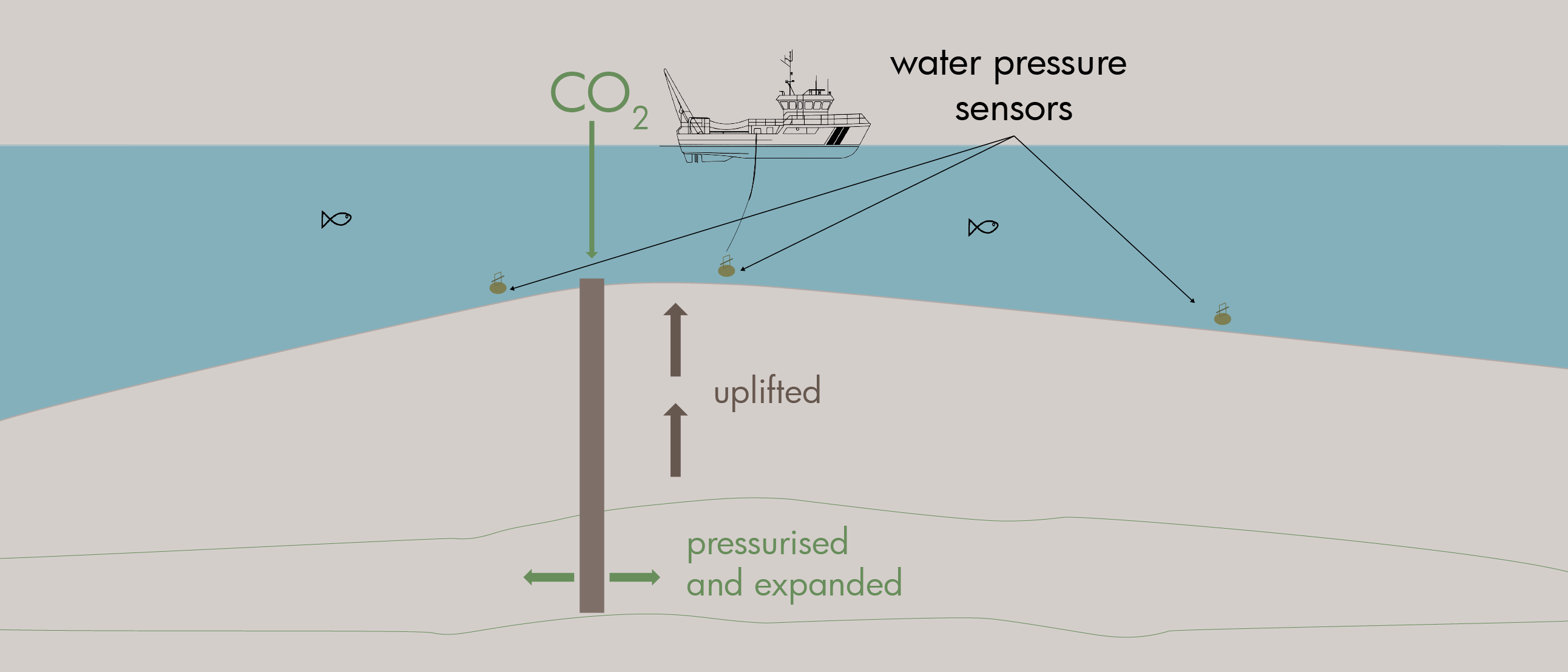
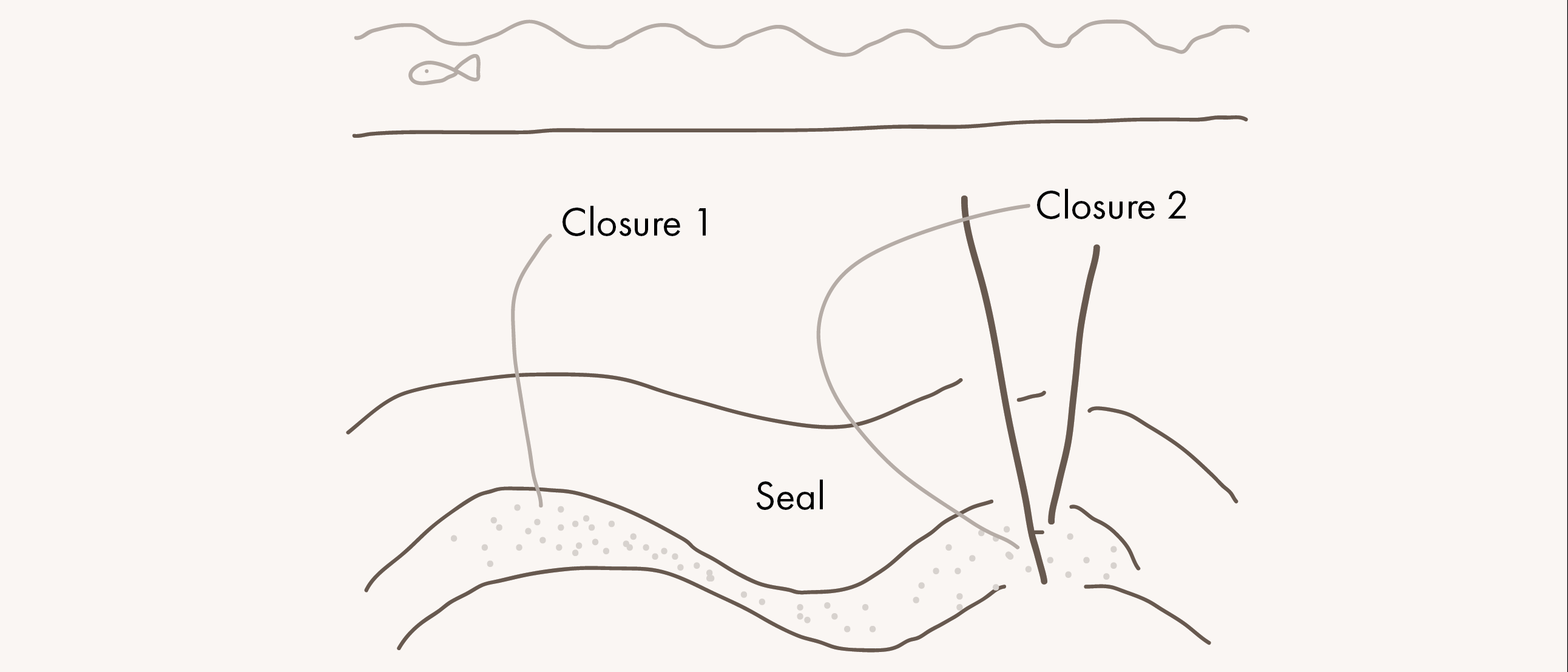
The main benefit of employing basalts for CO2 sequestration is the rapid timescales for CO2 conversion into solid form, which is in the order of months to years as opposed to the thousands or millions of years, if at all, anticipated for most traditional sedimentary storage systems. By using basalts for CO2 sequestration, the risks associated with unexpected plume migration and long-term seal integrity can, therefore, be avoided.
The conventional energy sector has rarely targeted basalts specifically, and our knowledge of these sequences as reservoirs is still in its infancy. But that may be set to change.
With pilot projects in the USA and Iceland, where CO2 has successfully been injected into basalts to test the process of mineralization, these extrusive rocks have become more important in the discussion of permanently storing CO2 in the subsurface, especially in areas where suitable sedimentary storage is not available.
Economically speaking, the best places to store CO2 are close to high CO2 emitters, which may not always be close to young, reactive volcanic sequences or safe sedimentary storage systems that are appropriate for conventional storage. For this reason, it is critical to understand whether CO2 storage is feasible for the range of geology available to us. Furthermore, young and relatively fresh basalts are not ubiquitous, and the reservoir properties of older basalts with more complex or protracted burial histories is not well understood. To form a better understanding of the variability and heterogeneity of basaltic reservoirs, I am studying the reservoir properties of basalts of different ages from the UK and abroad in terms of porosity, permeability and mineralogy to investigate their suitability for CO2 disposal.
It is estimated that the cost of CO2 storage in basalt is currently between $10- 30/tCO2. However, a number of variables, including the pH, temperature, and availability of reactive pore surfaces in the system, have a significant impact on the cost and rate of storage in a particular sequence. Therefore, to assess the suitability of a sequence for CO2 storage from both geological and economic perspectives, I am looking to understand the local vertical and lateral variations in mineralogy and permeability distributions and understand their effect on reservoir quality.
Exploring the feasibility of applying basalt storage techniques in other regions of the world also requires an assessment of other selection criteria, such as proximity to CO2 source, and the availability of transport infrastructure and water. For example, the Carbfix project in Iceland currently requires 22 tonnes of water to dissolve one tonne of CO2. Therefore, implementing similar projects on other regions of the world requires consideration to the availability of water and the potential costs associated with transporting that water to the injection site.