The petroleum system consists of source rock, migration pathways, reservoir, seal, and trap. Source rocks represent the hydrocarbon kitchen (generation) in a sedimentary basin. The source rock is also the first logical step to assess a petroleum system, for no matter how excellent a reservoir or a trap may be, without an effective source rock there would be no petroleum charge into the basin.
Searching for Good Source Rocks
Although there is evidence for non-organic methane (notably, gas emissions from volcanic eruptions), the majority of petroleum geochemists and geologists rightly regard the hydrocarbons we produce from sedimentary basins to be of organic origin. One line of evidence is oil shale, which contains plenty of organic matter (kerogen) but since the rocks were never deeply buried, the kerogen is thermally immature, and upon heating (‘cracking’), it transforms to simpler hydrocarbon molecules, similar to those found in petroleum reservoirs. Source rock is the mother of all oil and natural gas reserves.
Fine-grained, clay-rich sedimentary rocks including mudstone, shale (platy mudstone), marl (calcareous mudstone), limestone, and coaly rocks (especially for natural gas) are usually considered to be possible source rocks because coarse-grained sediments are too porous and permeable to retain organic matter. Moreover, organic matter tends to adsorb onto mineral surfaces of clay-rich sediments which, being fine-grained, have a larger mineral surface area per unit of rock volume. Marine environments in which fine-grained mudstone and clay-rich limestone are deposited coincide with the habitat and burial site of plankton biomass (which contribute 90% of organic matter to petroleum source rocks). Indeed, ocean waters, biota and rocks are the largest sink of carbon on Earth.
A ‘good’ source rock that yields commercial volumes of petroleum must have a high organic content and have compounds of both carbon and hydrogen molecules (otherwise, we end up with coal). It must be thermally mature through burial, have considerable thickness and lateral extent, and should be able to expel the generated oil.
A given shale formation will have different source-rock characteristics and ranking in various locations in a basin, and needs to be studied in detail.
Source Rocks in E&P
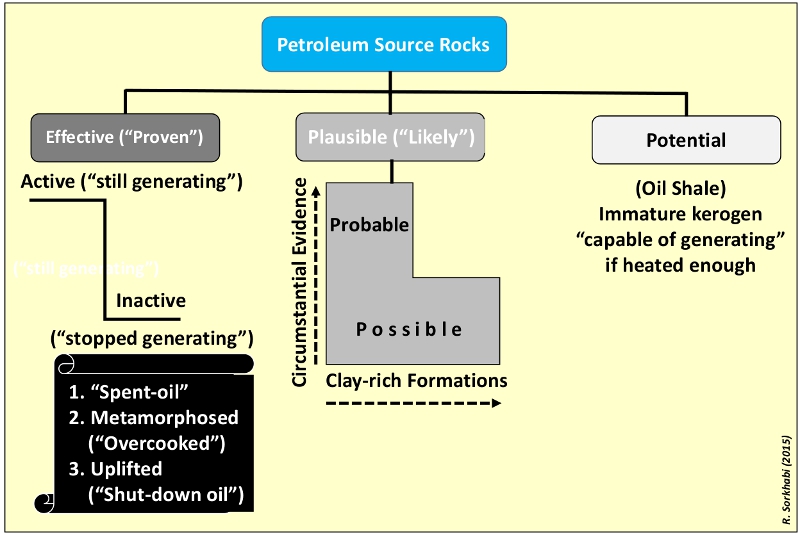 A classification of source rocks based on their probability of performance. (Source: Rasoul Sorkhabi)Despite its fundamental importance, the source was the last component of the petroleum system to be investigated scientifically, and petroleum geology textbooks of the 1920s–50s dealt mostly with reservoirs and traps. The first petroleum geochemistry books appeared in the late 1970s, including Petroleum Formation and Occurrence by B.P. Tissot and D.H. Welte and Petroleum Geochemistry and Geology by John Hunt.
A classification of source rocks based on their probability of performance. (Source: Rasoul Sorkhabi)Despite its fundamental importance, the source was the last component of the petroleum system to be investigated scientifically, and petroleum geology textbooks of the 1920s–50s dealt mostly with reservoirs and traps. The first petroleum geochemistry books appeared in the late 1970s, including Petroleum Formation and Occurrence by B.P. Tissot and D.H. Welte and Petroleum Geochemistry and Geology by John Hunt.
Because source rocks lie in the deeper parts of the basin they are not easily accessible for sampling and analysis. Analytical techniques for source rock geochemistry are also relatively recent: RockEval (pyrolysis) and vitrinite reflectance techniques were developed in the 1970s, and basin modelling software for source rock maturity and petroleum generation appeared in the 1980s.
The history of the petroleum industry since the mid-19th century may be viewed as the rise of four waves in exploration and production: onshore; nearshore and shelf; deepwater; and shale. For most of this history, exploration onshore and nearshore was primarily based on locating seeps and trap structures (so-called ‘wildcatting’). In deepwater basins the source rock is the last layer to be drilled, so we still have little data for deepwater source rocks.
The significance of source rock knowledge in exploiting shale plays should not be underestimated. Even though producing from these low-permeability rocks requires direct drilling, hydraulic fracturing and mapping of fracture sweet spots, at the resource level, the efficiency of shale plays is controlled by their geochemical, mineralogical, depositional and thermal maturity properties.
From Sunlight to Kerogen
Carbon (from Latin carbo, charcoal), with atomic number six, is the fourth-most abundant element (0.46% by mass) in the Milky Way galaxy, after hydrogen, 74%, helium, 24% and oxygen, 1.04%. Carbon, like many other elements, was created inside previous stars in the universe by nucleosynthesis. It constitutes no more than 0.18% of Earth’s crust.
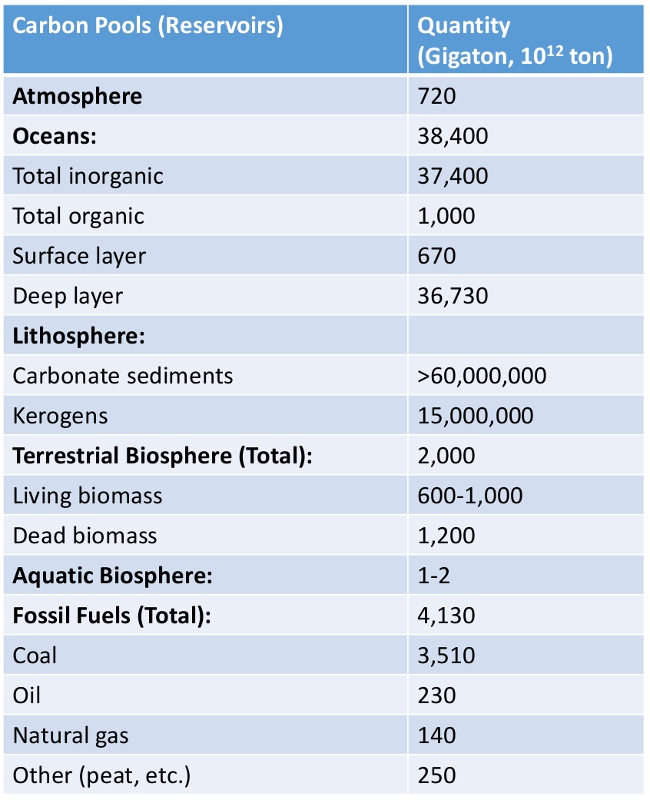 Carbon budget on Earth. (Source: The Global Carbon Cycle, Falkowski et al., Science 13 Oct. 2000_Before oil and gas are generated in the source rock, there is a long pathway for organic matter to be produced, accumulated and preserved in the basin. Organic matter dispersed in sediments is called kerogen (Greek: keros, wax, and gen, birth), a mixture of complex carbon-based chemical compounds that are insoluble in normal organic solvents because of their high molecular weights. The term kerogen was introduced by the Scottish chemist Alexander Crum Brown in 1906. Note that fresh organic matter in unconsolidated sediments up to 100m deep is not regarded as kerogen.
Carbon budget on Earth. (Source: The Global Carbon Cycle, Falkowski et al., Science 13 Oct. 2000_Before oil and gas are generated in the source rock, there is a long pathway for organic matter to be produced, accumulated and preserved in the basin. Organic matter dispersed in sediments is called kerogen (Greek: keros, wax, and gen, birth), a mixture of complex carbon-based chemical compounds that are insoluble in normal organic solvents because of their high molecular weights. The term kerogen was introduced by the Scottish chemist Alexander Crum Brown in 1906. Note that fresh organic matter in unconsolidated sediments up to 100m deep is not regarded as kerogen.
We often think of solar power as radiation from the sun that directly reaches Earth, but fossil fuels are also solar energy stored in rocks because the sun’s radiation (electromagnetic force) is involved in the production of organic carbon. Fossil fuels are an important component of the carbon cycle on Earth, which describes the interlocking circuits of carbon reservoirs (pools) and exchanges (fluxes) in the atmosphere, biosphere, hydrosphere and lithosphere.
Solar radiation that reaches the top of Earth’s atmosphere has been measured by satellites to be 1,361 W/m2. This average annual ‘solar constant’ amounts to a flux of 173,000 TW (173 x 10-12) of solar radiation for the whole Earth. It includes infrared (50%), visible (40%) and ultraviolet light (10%). About 30% of solar radiation is reflected back by the atmosphere, clouds and land as shortwave radiation; the remaining 70% is absorbed by land, oceans and atmosphere for various processes, but is eventually radiated back to space. Only 40 TW (0.023%) of solar radiation is involved in photosynthesis, the source of organic matter.
The Carbon Cycle
Photosynthesis and respiration are probably the most familiar processes of the carbon cycle to us. Plants utilise sunlight, carbon dioxide and water to produce food (carbohydrate molecules such as sugars) and oxygen. Animals, in turn, depend on plants (or other animals) for food, and their respiration involves inhalation of oxygen and exhalation of carbon dioxide. Photosynthesis and respiration occur in both terrestrial and aquatic ecosystems, the difference being that land plants and animals utilise the atmospheric carbon dioxide and oxygen while marine organisms (phytoplankton and zooplankton) get these substances from sea water. Note that sunlight also penetrates the upper 200m of oceans – the photic zone, where about 90% of marine organisms live.
Photosynthesis and respiration are parts of the ‘fast carbon cycle’. The ‘slow carbon cycle’, which operates on longer time periods, includes the chemical weathering (thus absorbing atmospheric carbon dioxide) and erosion of rocks, and the transport of organic matter by rivers to oceans, as well as the plate tectonic subduction of carbonate rocks and release of carbon dioxide from volcanoes. The slow carbon cycle carries terrestrial carbon to oceans where it is incorporated in marine organisms or deposited as carbonate rocks on the sea floor.
Since high levels of biological activity increase the production of organic matter, the supply of nutrients – phosphates and nitrates – for organisms is another condition favourable for deposition of rich source rocks. Continental margins with sediment-laden rivers naturally have abundant nutrients. Marine ‘upwelling zones’ are also important providers of nutrients to stimulate the growth of phytoplankton populations (primary producers of organic matter). Upwelling is the rise of colder, nutrient-rich water from the ocean bottom to the sea surface, the nutrient content being derived from decomposition of dead plankton. Marine upwelling is caused by various mechanisms. In coastal areas, for example, where winds move parallel to the shore and surface waters move offshore perpendicular to the wind direction, deeper and colder water upwells to the surface. In equatorial coasts, the eastward trade winds drive away surface waters from the western coastline of the continents, thus inviting deeper colder water to rise and replace the surface waters.
Anoxic Environments and Sedimentation
At present, total production of organic matter in the world’s oceans amounts to 50 billion tons a year, but over 99% of this is broken down by oxidation, microbial activity or physicochemical changes in water column. Therefore, only 0.01–0.001% of organic matter is preserved in sedimentary rocks as kerogen or fossil fuels as part of the ‘slow carbon cycle’. This indicates the preciousness of petroleum resources formed over millions of years, hence their categorisation as non-renewable resources on the human timescale, although petroleum generation is a continuous, renewable process on the geological timescale.
When organic matter combines with oxygen, the reaction produces carbon dioxide and oxygen molecules – the reverse of photosynthesis, and also what happens when we burn fossil fuels. Oxygen thus acts as a double-edged sword: it helps to produce organic matter, yet also destroys it. Therefore, for organic matter to be preserved in sedimentary rocks, an anoxic environment (O2 <0.2 mL/L) is required. Anoxic conditions are found in lakes with thermally stratified waters (warm water over cold water), in barred nearshore basins with salinity stratification (light water atop dense water), and continental margins at low latitudes (and close to upwelling zones) where water depths of 200–1,500m (below the photic zone) are deficient in oxygen.
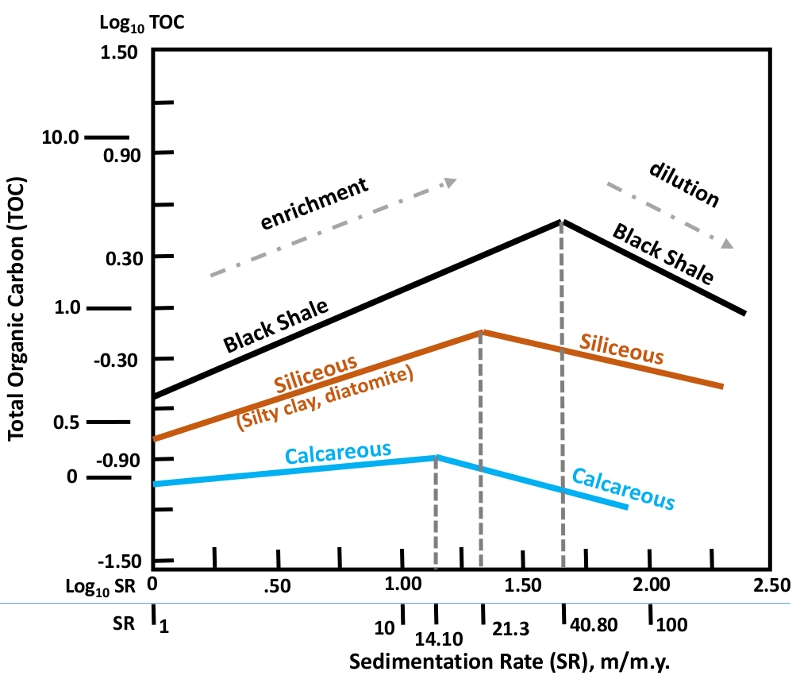 Relationships between sedimentation rate and total organic carbon for various marine rocks. Data for Jurassic – Recent sediments recovered by the Deep Sea Drilling Project (Johnson Ibach, 1992, AAPG Bulletin, 66: 170-188).Obviously, oceanic anoxic events, during which vast parts of the world’s oceans were depleted in oxygen, favour the deposition of organic-rich source rocks. Such events require high levels of atmospheric carbon dioxide (from volcanic eruptions), warm climates (mainly due to atmospheric greenhouse effect), sluggish ocean currents, and euxinic (sulphidic) water chemistry (from volcanic outgassing). Extreme volcanic activity also supplies nutrients that stimulate planktonic productivity in the oceans. Global anoxia have been recorded in the Jurassic and Cretaceous periods – these rocks account for 70% of the world’s petroleum reserves. Several intervals of anoxia have also been found throughout the Palaeozoic era. Intense global anoxia have also been linked to marine mass extinctions because of climatic change and toxic (H2S-rich) waters.
Relationships between sedimentation rate and total organic carbon for various marine rocks. Data for Jurassic – Recent sediments recovered by the Deep Sea Drilling Project (Johnson Ibach, 1992, AAPG Bulletin, 66: 170-188).Obviously, oceanic anoxic events, during which vast parts of the world’s oceans were depleted in oxygen, favour the deposition of organic-rich source rocks. Such events require high levels of atmospheric carbon dioxide (from volcanic eruptions), warm climates (mainly due to atmospheric greenhouse effect), sluggish ocean currents, and euxinic (sulphidic) water chemistry (from volcanic outgassing). Extreme volcanic activity also supplies nutrients that stimulate planktonic productivity in the oceans. Global anoxia have been recorded in the Jurassic and Cretaceous periods – these rocks account for 70% of the world’s petroleum reserves. Several intervals of anoxia have also been found throughout the Palaeozoic era. Intense global anoxia have also been linked to marine mass extinctions because of climatic change and toxic (H2S-rich) waters.
Organic matter in ocean waters exists in three different forms: particulate matter; in solution; and in colloidal form. Particulate organic matter may directly drop to ocean bottom, while dissolved organic matter may be adsorbed onto clay grains, which then sink slowly through the water. Colloidal organic matter flocculates to particulates before sinking.
Organic matter that reaches the sea floor is buried in sediments. It is intuitive to consider that more sedimentation favours the deposition of rich source rocks not only because of the greater volume of sediments, but also because rapid sedimentation and burial impedes oxidation processes, thus preserving organic matter. This is true only to some degree, because high sedimentation rates actually lead to dilution of organic matter in sedimentary rock formations.
A Summing-up
Basins with restricted water circulation and stratified water column will preserve more organic carbon in sediments and produce good source rocks. To sum up our discussion in this article, the following relationship is quoted from a seminal paper by Kevin Bohacs et al. (2005, SEPM Special Publication 82, pp. 61-101):
Organic-matter enrichment = Production – (Destruction + Dilution)
Production of organic matter is a function of photosynthesis (sunlight, water, carbon dioxide and nutrient supply) and plankton population. Destruction is related to exposure time to oxidants. Dilution is a function of clastic sedimentation rate and production of biogenic material (silica or carbonate).
In short, ‘significant enrichment of organic matter occurs where organic-matter production is maximised, destruction is minimised, and dilution by clastic or biogenic material is optimised.’