In Part II we look at Arrhenius’ seminal 1896 paper and see how it relates to blackbody radiation and absorption of infrared radiation by the atmosphere, taking a closer look at his model of the greenhouse effect.
Recent Advances in Climate Change Research: Part I – Blackbody Radiation and Milankovic Cycles
“Humanity stands … before a great problem of finding new raw materials and new sources of energy that shall never become exhausted. In the meantime we must not waste what we have, but must leave as much as possible for coming generations.”
Svante Arrhenius (1859–1927)
Svante Arrhenius is often mentioned as one of the first scientists to couple atmospheric CO2 to the Earth’s temperature. Following on from Part I, where we introduced blackbody radiation and Milankovich cycles, here we look at Arrhenius’ seminal 1896 paper and see how it relates to blackbody radiation and absorption of infrared radiation by the atmosphere. It is surprising to see how close his 1896 predictions are to today’s advanced climate models.
Introducing Svante August Arrhenius
Figure 1: Svante Arrhenius. Source: Nobel Foundation (Public domain).
Svante August Arrhenius was born in Vik Castle close to Uppsala in 1859. He is best known for his theory of electrolytic dissociation and his model of the greenhouse effect. In 1903 Arrhenius was awarded the Nobel Prize for Chemistry. He learned to read at the age of 3, and at 8 he entered 5th grade at the local cathedral school. In 1884 he delivered his PhD thesis on conductivities of electrolytes, 150 pages, to the University of Uppsala. The thesis was not well received by his professors, and he got a fourth-class degree that was later re-classified to third-class. Part of the work in his PhD turned out to be the basis for the Nobel Prize for Chemistry. Around 1900 Arrhenius was involved in setting up the Nobel prizes, and he was a member of the Swedish Nobel committee for physics and chemistry for the rest of his life. He was elected a member of the Swedish scientific academy in 1901, against heavy protests because it was thought he was using his position on the Nobel committee to promote friends and stop enemies from receiving the award.
Radiation from Blackbodies: 1870–1910
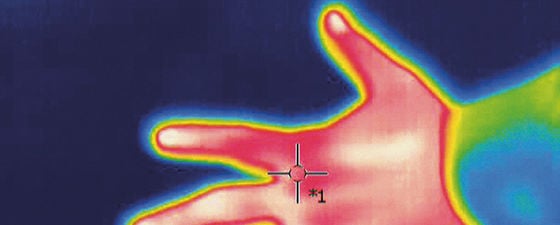
Figure 2: Thermal photo of a hand, exploiting blackbody radiation to determine the temperature of the various parts of the body. Source: Jarek Tuszyński / CC-BY-SA-3.0.
When Svante Arrhenius wrote his famous 1896 paper On the influence of carbonic acid in the air upon the temperature of the ground, he was using knowledge and input from colleagues related to infrared radiation from blackbodies. (A blackbody is an idealised physical body that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence – see Part I in GEO ExPro Vol. 16, No. 2.)
The empirical radiation law derived by Stefan and Boltzmann, stating that the total energy radiated per unit surface area of a blackbody in unit time, or the radiated energy flux, is proportional to the body temperature (measured in Kelvin; 273.15 K = 0 °C) into the fourth power, was known:
U = σT4 [Wm–2} (1)
where σ = 5.67 x 10–8 Wm–2K–4 is Stefan-Boltzmann’s constant. This means that if you double an object’s temperature, the amount of energy it releases increases by a factor of 16. Energy flux has dimensions of energy per time per area, and the SI units to measure it are joules per second per square metre, watts per square metre. The radiated energy flux is also known as the blackbody irradiance. To find the total absolute power of energy radiated for the blackbody object we multiply by the surface area A; then P = AU.
Figure 3: Ludwig Boltzmann (at the table) with co-workers in 1887. Svante Arrhenius is standing on his left. Source: Universität Graz (Public domain).
Josef Stefan derived this law in 1879 based on experimental data obtained by John Tyndall. In 1884 Stefan’s student, Ludwig Boltzmann, showed that this fourth order power law can be derived directly from thermodynamics. Svante Arrhenius had regular contacts with Boltzmann and his coworkers in Austria, meaning that blackbody radiation and the physics behind this was well understood by Arrhenius when he wrote his 1896 paper. When we use the term ‘light’ in this context we mean the whole electromagnetic spectrum (see Part I).
However, the direct coupling between Stefan-Boltzmann’s law and quantum mechanics was not revealed until Max Planck formulated his radiation law in 1900. Planck showed that the power emitted per unit projected area of a blackbody at temperature T, into a unit solid angle, per wavelength λ , is
Here, c is the speed of light, h is Planck’s constant, and k is Boltzmann’s constant. The quantity Bλ is referred to as the spectral radiance. Its dimension includes the steradian unit. It is simply a unit solid angle (see Part I).
Radiance is Radiance is the power emitted (or received) by a given surface, per unit solid angle per unit area. To find the radiance, one integrates the Planck equation (2) over all wave numbers,
The total radiated power per unit area, called the radiant exitance or irradiance, with symbol U, is found by further integrating B with respect to a solid angle over the hemisphere into which the surface radiates. Since blackbody radiance is independent of the direction of emission, irradiance emitted by a blackbody is:
The pile of constants in front of the temperature is Stefan-Boltzmann’s constant.
If we multiply the radiant exitance by the entire surface area of the body, we can find the total amount of power given off.
The empirical equation derived in 1879 was found to be of a much more fundamental nature 21 years later, as a result of the work of Max Planck, including quantum mechanics. Max Planck received the Nobel Prize for Physics in 1918. Equations 1 and 2 above contain a lot of information and represent a giant step in physics. By introducing quantum theory, Planck showed that it is possible to predict the ‘colour’ (or wavelength) distribution if we know the temperature of the blackbody, and vice versa. Furthermore, he showed that there is this fundamental relation between thermodynamics and quantum theory and that the apparent empirical Stefan-Boltzmann’s constant is directly related to quantum theory (Planck’s constant).
Radiating Blackbodies
Figure 4: Blackbody radiation curves, showing at which wavelength bodies of a certain temperature emit their maximum intensity.
A blackbody object radiates at all wavelengths. The wavelength that corresponds to the peak of spectral irradiance for a given temperature is found by taking the derivative of the irradiance with respect to wavelength, and setting the resulting expression to zero. Trust us: the solution gives the Wien displacement law:
λpeak = b/T
where b = 0.0029. This relation makes it possible to compute the temperature of a blackbody by measuring the wavelength of peak spectral radiance. If λpeak = 0.5 x 10–6m (500 nm), then T = 5,800K. This corresponds to green light in the middle of the visible spectrum.
An example of blackbody radiation from earth and moon is shown in the graph in Figure 4, where we have also included the most relevant absorption band for CO2, ranging from approximately 12 to 18 μm. Water vapour has more extensive and slightly different absorption bands.
Anything that heats up on Earth will release long wavelength (infrared) radiation. The graph shows blackbody radiation from a person (310.15 K), the Earth (288 K) and the moon (255 K), using Planck’s radiation law. On Earth, part of the incoming sunlight is reflected by the atmosphere and the surface. Most of the sunlight, however, is absorbed by the surface, which is warmed. Infrared radiation is then emitted from the surface. Visible light ranges from 0.4–0.7 μm (bars on the left of the graph, Figure 4), and the infrared range is between 0.7 and 1,000 μm, hence the dominant radiation from the Earth and the moon is within the infrared band. The typical absorption band for CO2 is indicated by the Valentine colour band, ranging from approximately 12 to 18 μm. There is also a CO2 absorption band between 4 and 4.5 μm.
A human body temperature of 310.15 Kelvin corresponds to a blackbody radiation wavelength of about 9.3 μm, which is in the infrared part of the spectrum. This infrared radiation of human bodies is the principle behind ‘night vision’ goggles: they convert infrared light to visible light, allowing the wearer to see warm bodies glowing in infrared light.
Arrhenius and Climate Change
In relation to climate research Arrhenius is known for the following equation, often referred to as the Arrhenius forcing law:
ΔF = α ln(C/C0)
where C0 is the concentration of CO2 at a reference time, and C the current concentration of CO2 in the atmosphere, α is a constant and ΔF is the radiative forcing, which is related to the temperature increase, ΔT ~ ΔF. The general rule from Arrhenius’ model is that if the quantity of CO2 increases or decreases, then temperature will increase or decrease. He predicted that a doubling of CO2 in the atmosphere would lead to temperature increases of 3 to 4°C.
In his 1896 paper, Arrhenius uses radiation data obtained by Langley on atmospherical absorption. The first sentence in this paper reads: “A great deal has been written on the influence of the absorption of the atmosphere upon the climate. Tyndall in particular has pointed out the enormous importance of this question.” Langley had published a paper in 1890 entitled The Temperature of the Moon where he had estimated the average temperature of the moon to be about 45°C. Today we know that it is significantly lower, close to 247 K (-20 °C).
Figure 5: Table VII in Arrhenius’ paper from 1896. He estimated changes in the Earth’s temperature for a doubling of CO2 (middle column) and for a reduction to 67% of the present value in 1896 (left column) as a function of latitude. From the middle column we observe a temperature increase of 4–5°C at the equator and up to 6° closer to the poles. In this paper he does not suggest that burning fossil fuels will cause global warming, although he does suggest this in later work.
Arrhenius’ idea was to use the absorption data obtained by Langley’s measurements of the radiation from the moon to estimate the influence of CO2 (denoted by K in his paper) and water vapour (denoted by W) on the Earth’s surface temperature. Arrhenius roughly divided the observations into four groups, where K = 1.21; 0.36; 2.21 and 0.86 and W = 1.33; 1.18; 2.22 and 2.34. Based on this, he made a table (Figure 5) in which he estimates temperature changes as a function of latitude for both increase and decrease of CO2. Although the actual numbers and the assumptions made are not regarded as valid today, it is interesting to observe that his simplified analysis predicts that the warming increases with latitude, and that a doubling of atmospheric CO2 leads to 5–6 degrees of warming – not far from the estimates of modern climate models.
In the paper, there is a separate section discussing geological consequences. He mentioned the lively discussions at the Physical Society of Stockholm on potential causes for the ice ages, and proposed that variation in CO2 level might be one cause.
Further Reading on Climate Change Research
More articles from the “Recent Advances in Climate Change Research” Series:
Part I – Blackbody Radiation and Milankovic Cycles
Martin Landrø and Lasse Amundsen, NTNU / Bivrost Geo
Geoscience will probably play an important role in mitigating carbon dioxide emissions. In part one of this series, we discuss some history and physics behind the topic of climate change including the concepts behind blackbody radiation and Millankovic Cycles.
This article appeared in Vol. 16, No. 2 – 2019
Part III – A Simple Greenhouse Model
Martin Landrø and Lasse Amundsen, NTNU/Bivrost Geo
What would the temperature of Earth be without the atmosphere? By using simple physical models for solar irradiation and the Stefan-Boltzmans law for blackbody radiation, we can estimate average temperatures with and without atmosphere.
This article appeared in Vol. 16, No. 4 – 2019
Part IV – Challenges and Practical Issues of Carbon Capture & Storage
Martin Landrø, Lasse Amundsen and Philip Ringrose
The basic idea behind CCS (Carbon Capture and Storage) is simple, but what are the main challenges and practical issues preventing a more global adoption of this method?
This article appeared in Vol. 16, No. 5 – 2020
Part V – Underground Storage of Carbon Dioxide
Eva K. Halland, Norwegian Petroleum Directorate. Series Editors: Martin Landrø and Lasse Amundsen, NTU/Bivrost Geo
By building on knowledge from the petroleum industry and experience of over 23 years of storing CO₂ in deep geological formations, we can make a new value chain and a business model for carbon capture and storage (CCS) in the North Sea Basin.
This article appeared in Vol. 16, No. 6 – 2019
Part VI – More on the Simple Greenhouse Model
Lasse Amundsen and Martin Landrø, NTU/Bivrost Geo
We continue the discussion of the simple greenhouse model introduced in Part III.
This article appeared in Vol. 17, No. 1 – 2020