Today found widespread on Earth wherever stability conditions are met and an ample supply of natural gas is present, methane hydrate was originally thought to occur in the outer region of the solar system where temperatures are low and water ice is common. We look into the possibilities of gas hydrates on Mars and elsewhere in outer space.
Science is a wonderful thing if one does not have to earn one’s living with it. Albert Einstein
Gas clathrate hydrates are ice-like compounds in which guest molecules of gas such as carbon dioxide (CO2), methane (CH4), ethane (C2H6), propane (C3H8), and hydrogen sulfide (H2S) are trapped within cage-like structures of water ice. Gas hydrates are stable at elevated pressures and temperatures compared to frozen water.
Mars, the ‘red planet’, has intrigued humans for thousands of years. It is named after the Roman god of war because of its red hue and that colour’s association with blood. Mars has polar ice caps, made of water ice, surrounded by frozen carbon dioxide which expands and contracts with the seasons. On average, the temperature on Mars is about -60°C. Near the equator temperatures vary from a warm summer 20°C in the day to an arctic -70°C in the night, while in winter temperatures near the poles can drop down to -125°C.
CH4 on Mars?
 NASA’s Mars rover Curiosity sampling gas on Mars. (Source: NASA/JPL-Caltech/MSSS)The debate over if and how much methane is present on Mars has been long-running. In 2004, several research teams announced that they had spotted traces of methane, which could have come from microbes or geological activity, or been delivered as extraplanetary methane by crashing comets – all thrilling possibilities. However, the observations were indirect and uncertain.
NASA’s Mars rover Curiosity sampling gas on Mars. (Source: NASA/JPL-Caltech/MSSS)The debate over if and how much methane is present on Mars has been long-running. In 2004, several research teams announced that they had spotted traces of methane, which could have come from microbes or geological activity, or been delivered as extraplanetary methane by crashing comets – all thrilling possibilities. However, the observations were indirect and uncertain.
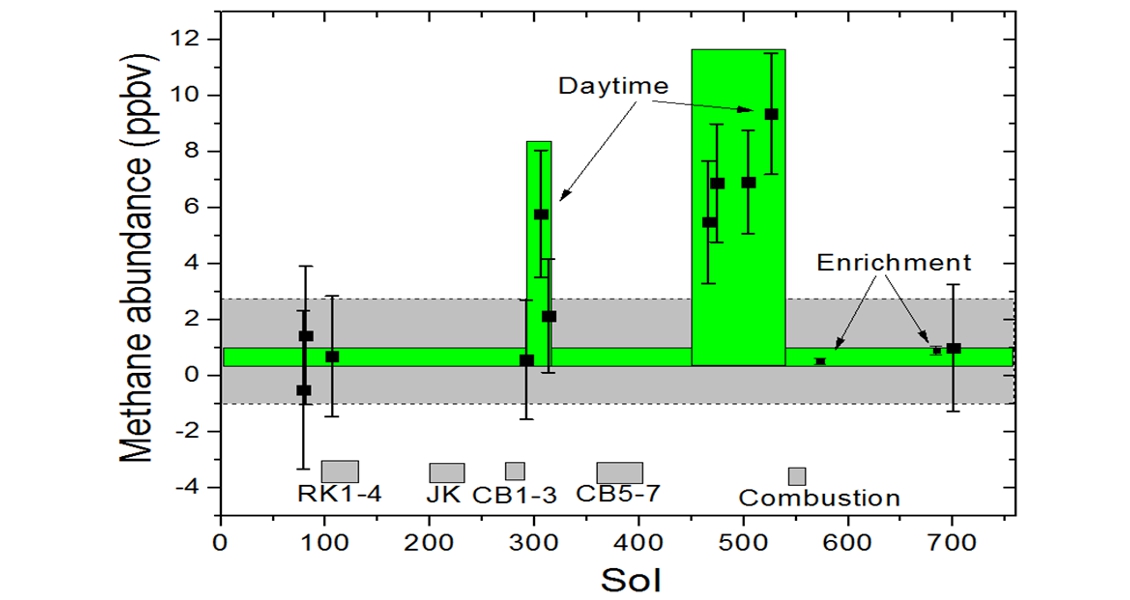
Then, at a meeting of the American Geophysical Union in December 2014, researchers announced that NASA’s Mars Curiosity rover had found a small amount of methane wafting over its landing spot in Gale Crater. Over the course of 605 Martian days (Mars’ solar day is only about 2.7% longer than Earth’s), Curiosity measured background methane levels at 0.7 parts per billion – significantly lower than earlier claims. Occasionally, however, the rover recorded atmospheric levels of methane as high as roughly 7 parts per billion – a tenfold rise. Thus, Mars seems to be actively both producing and releasing methane.
However, the source, or even whether it is biological or geological, is unknown. For example, a possible biological origin could be microbes living in the groundwater below a permafrost zone, whose waste methane could percolate up and leak out. Alternatively, a geological source for the methane could be buried volcanic rocks rich in the mineral olivine, chemically reacting with water to produce methane. Another possible origin could be that the methane is escaping from possible ancient clathrates, buried deposits of methane ice formed long ago by one of the other two mechanisms.
CH4 Hydrate on Mars?
In the subsurface of Mars CO2 hydrate, in addition to CH4, is predicted to be stable if the guest molecule gas is present in requisite concentrations.
CO2 (commonly known as ‘dry ice’) is an abundant volatile on Mars, comprising 90% of the planet’s atmosphere, the remaining 10% being composed of water vapour, nitrogen, and other gases. It is possible for CO2 hydrates to exist just below the surface of Mars because of the very cold temperatures, and it has been estimated that the gas hydrate stability zone could extend more than a kilometre beneath the surface.
The surface temperature of Mars has seasonal changes which may cause continuous variations of atmospheric composition due to hydrate formation and dissociation. CO2 hydrates may exist on the Martian surface in the impressive polar caps and also in the atmosphere in the form of clouds. If they are present in the former, then the cap will not melt as readily as it would if it consisted only of water ice, because of the clathrate’s lower thermal conductivity, higher stability under pressure, and higher strength, when compared to pure frozen water.
Surface Features on Mars
 This set of images was taken by the High Resolution Imaging Science Experiment (HiRISE) camera in 2010 and 2013. A new channel is shown forming on the Martian slope. New research reveals that sand propelled on a cushion of CO2 gas could be responsible for slicing into the red planet’s surface. (Source: NASA/JPL-Caltech/Univ. of Arizona)It has been proposed that the decomposition of CO2 hydrate could play a prominent role in the ‘terraforming’ processes on Mars, and many of the observed surface features may be partly attributed to it.
This set of images was taken by the High Resolution Imaging Science Experiment (HiRISE) camera in 2010 and 2013. A new channel is shown forming on the Martian slope. New research reveals that sand propelled on a cushion of CO2 gas could be responsible for slicing into the red planet’s surface. (Source: NASA/JPL-Caltech/Univ. of Arizona)It has been proposed that the decomposition of CO2 hydrate could play a prominent role in the ‘terraforming’ processes on Mars, and many of the observed surface features may be partly attributed to it.
Martian gullies seen on the surface of the planet, for instance, have long been a mystery. The gullies are small, incised networks of narrow channels and their associated downslope sediment deposits. It has been argued that these were formed not by liquid water but by CO2, since the present Martian climate does not allow liquid water to exist on the surface in general and recent observations now indicate the gullies are indeed primarily formed by the seasonal freezing of CO2, not liquid water. The polar surface, which is covered by layers of sand and dust, is made up of frozen CO2 and water. In the spring, when the sun warms up the polar slopes, the frozen gas and water don’t melt. Instead the gases sublimate (i.e. change from solid directly to gas, without pausing to form a liquid). The vapour lifts the sediment off the surface, reducing the friction and allowing the sand to move more easily. This sand is moved down steep slopes, gashing the surface like water running downhill. The temperature at which CO2 changes from solid to gas is -78.5°C.
We end this part of the story by noting that the possibility of extracting water from hydrates could help stimulate attempts at human colonisation of Mars. But to ‘terraform’ Mars, or turn the planet into a smaller version of Earth, unfortunately the first settlers will have to live below ground to protect themselves from cosmic rays.